Having studied the motion of dissolved elements, corresponding to potassium and glucose iodide, and monitoring adjustments within the weight in quite a lot of concentrations of sucrose, the experiment seeks to reveal the foundations of passive shipping and because the focus gradients throughout the membranes.
This experiment examines how quite a lot of concentrations of solvents impact the processes of osmosis and diffusion within the semi -permeable membrane (dialysis tube), inspecting the motion of water, solvents and ensuing adjustments within the traits of the answer and weight in time.
Creation
Osmos and diffusion are basic processes in cell shipping, taking part in crucial position in keeping up homeostasis and offering the essential biochemical purposes (Alberts et al., 2014). Each are kinds of passive shipping, permitting molecules to transport thru organic membranes with out power. Osmos is outlined because the motion of water molecules throughout the semi -permeable membrane from the realm of a decrease focus of the mortar into the realm of the next focus of the dissolved substance till equilibrium is accomplished. To the contrary, diffusion comprises the motion of dissolved debris from the next focus to a decrease focus house (Binod, 2024).
Osmosis throughout the cellular membrane
Working out the dynamics of those processes is essential to check how the cells engage with the surroundings, soak up vitamins and do away with waste. There are 3 primary sorts of answers in the case of the osmosis: hypertensive, the place there are extra solvents outdoor the cellular than inside of, on account of which water flows from the cellular; hypotonic, the place there are fewer solvents outdoor the cellular, which results in water shifting into the cellular; And isotonic, the place the focus of dissolved elements is similar outside and inside the cellular, which doesn’t result in the loss of natural water motion (Binod, 2024).
On this experiment, the experiment makes use of dialysis programs, as they’re semi -permeable and constitute synthetic cells for finding out osmosis and diffusion. The speculation of the experiment lies in the truth that an answer of starch/glucose in a dialysis tube could have a metamorphosis in colour because of diffusion of potassium iodide. And the load of dialysis baggage will trade from the osmosis, relying at the concentrations of sucrose.
Fabrics and strategies
Steps for the learn about of diffusion and osmosis the use of a dialysis tube
Diffusion experiment:
- Get ready an expansion resolution:
- Fill a small glass with water.
- Upload 10 drops of potassium iodide to a tumbler to succeed in medium brown.
- Watch the diffusion:
- Write down the preliminary colour and look of a potassium iodide resolution in a tumbler.
- Stay up for a diffuse resolution, staring at the colour trade till the answer turns into yellow in every single place, which signifies whole diffusion.
- Get ready a dialy tube:
- Minimize a work of dialysis of the tube about 10 cm lengthy.
- The clamp one finish of the tube is dependable and go away the opposite finish open.
- Fill in dialysis tubes:
- Fill within the open finish of the dialysis tube of about two -thirds full of an answer of starch/glucose.
- Reliably press the open finish of the tube.
- Write down the preliminary observations:
- Write down the colour of each iodide potassium resolution in a tumbler and a starch/glucose resolution in a dialysis tube in desk 1.
- Dip the check strip of glucose in a tumbler resolution and write down the lead to desk 2.
- Position the dialysis tubes in a tumbler:
- Pricey the ready dialysis tube in a tumbler containing an answer of potassium iodide.
- Wait and apply:
- After half-hour, write down adjustments within the colour of each an answer of potassium iodide in a tumbler and an answer of starch/glucose inside of a dialysis tube in desk 1.
- Dip the check strip of glucose in a tumbler resolution once more and write down the lead to desk 2.
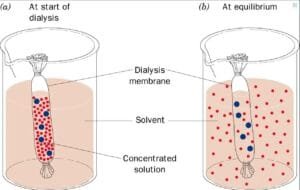 Experiment with dialysis
Experiment with dialysis
Osmoz experiment:
After 60 mins, write down the general weights of all dialysis cells in desk 3.
Get ready osmosis answers:
- Fill a small glass of about two -thirds full of a 25% resolution of sucrose.
- Fill a big glass in about two -thirds full of 1% sucrose resolution.
Get ready a dialy tube for synthetic cells:
- Get 4 portions of a soaked dialysis tube.
- Press one finish of each and every piece of dialysis tube with a clamp. Get the clips a, b, C and D.
Fill in a dialy tube:
- Open “Cell A” and fill it in about two -thirds full of 1% sucrose resolution, after which securely repair it.
- Fill within the “cell B” with a 1% resolution of sucrose, “cell C” with a ten% resolution of sucrose and “cell D” with a 25% sucrose resolution, reliably clamping each and every.
Write down the preliminary weights:
- Weigh each and every of the 4 dialysis cells and write down their preliminary weights in desk 3.
Dive dialysis cells:
- Position the “cell A” in a small glass with a 25% sucrose resolution.
- Position “Cells B, C and D” in a big glass with 1% sucrose resolution.
Wait and weigh:
- After quarter-hour, take away the cells from their corresponding glasses, quite dry them and weigh.
- Write down the brand new weight in desk 3.
- Go back the cells to their corresponding glasses.
Repeat the weighing procedure:
- Repeat the method of removing, drying, weighing and recording weights after half-hour, 45 mins and 60 mins.
Ultimate weights:
- After 60 mins, write down the general weights of all dialysis cells in desk 3.
 Diffusion and dialysis thru a dialysis package deal
Diffusion and dialysis thru a dialysis package deal
Effects
The result of the experiment reveal the diffusion of potassium iodide and the conduct of glucose within the dialysis tube, as proven within the following tables.
Desk 1.
An answer of potassium iodideStarch/glucose resolutionPreliminary colourYellowish brownThe overall colourClearpurple/Cloudy
Desk 2. Glucose check for diffusion
ColourIs glucose provide?Glucose check strip originallyTilnegativGlucose check strip on the finishBrown/Golce Glucose
The result of the osmosis experiment are demonstrated by way of the motion of dissolved elements within the dialysis tube and adjustments in weight, which signifies a hypertensive, hypotonic or isotonic nature of our environment.
Desk 3. Converting the load of dialysis cells relying at the time
0 mins (preliminary weight) (GM)Weight 10 mins (GM)Weight 20 mins (GM)half-hour of weight (GM)40 mins weight (GM)Cellular a24.1624.5323.9024.1224.65Cellular b19.2719.3119.3419.3019.33Cellular p24.6724.5824.3324.0123.79Cellular d28.5026.8725.6525.5724.78
Dialogue
The experiment demonstrated that potassium iodide spreads into an answer of starch/glucose in a dialysis tube, inflicting a colour trade and confirming a hit diffusion. As well as, the load of dialysis baggage various relying on their surrounding sucrose concentrations, which signifies the impact of the osmosis: the cells in hypertensive answers spent weight, whilst in isotonic answers remained strong.
The preliminary observations confirmed that the answer of potassium iodide started as yellowish -brown, and the starch/glucose resolution used to be white. After half-hour an answer of potassium iodide
It become transparent, and the starch/glucose resolution become red/cloud, which signifies the a hit diffusion of iodine into the tube.
The glucose check strip at first confirmed the colour of turquoise colour, which signifies the loss of glucose. On the finish of the experiment, the strip modified to brown/inexperienced, confirming the hint of glucose in an answer of a tumbler.
Adjustments in time in time point out quite a lot of solutions to the encompassing answers. Cellular A confirmed a slight build up in weight after 10 mins, however after that he hesitated, which signifies a hypertensive atmosphere in a tumbler. Cage B remained moderately strong, indicating an isotonic atmosphere. To the contrary, the cellular C demonstrated a steady weight reduction, whilst in cells D confirmed a vital lower, reflecting the lack of water from its high blood pressure. Dissolved is going from the next focus to a decrease focus to take care of homeostasis (Alberts et al., 2014).
The experiment had weaknesses, corresponding to variations in how neatly the dialysis tube allowed elements to move thru itself, which might result in asymmetric effects. As well as, to not keep watch over the temperature and depend on colour adjustments for measurements may just make the consequences much less dependable.
Basically, the consequences verify the speculation that diffusion happens all over the dialysis membrane and emphasizes the impact of osmotic force at the weight of dialysis of baggage.
Additional experiments
Long run experiments can learn about how the temperature impacts osmosis and diffusion, anticipating that upper temperatures will make those processes quicker. Shall we additionally take a look at other elements corresponding to salt or sugar to look how they modify the molecule.
Hyperlinks
Alberts B., Johnson A., Lewis J., Raff M., Roberts Okay. and Walter P. (2014). Molecular cellular biology (sixth ed.). Harland science.
GC, Binod. “Osmos and diffusion: differences and factors that affect them.” Word scienceApril 14, 2023. Internet. October 2, 2024. Osmosis and diffusion: variations and components affecting them
Mika, T.A., Klein, R.J., Bullerkhan, A.E., Connor, R.L., Plavik, L.M., White, Re,
Gosses, MW, Carter, Te, Andrews, Am, Maier, Jl, & Sidiq, F. (EDS.). (2024). Anatomy and body structure BIO 211 Laboratory Information (third ed.). Owens Public School.
GC, Binod. “Cell transport: passive and active mechanisms.” Word scienceSeptember 3, 2024. Internet. October 2, 2024. Cell shipping: passive and energetic mechanisms – clinical notes



